The national health spending in the US is expected to reach $6.4 trillion by 2028. The FDA regulates the healthcare industry in the United States. If your company uses medical device software, then its validation is your responsibility. FDA software validation ensures that the tools you use for product creation and distribution are up to mark.
Software validation should go beyond compliance standards. It is a prerequisite for any company that cares about upholding standards or ensuring the reliability of its software.
FDA software validation is a must, but it isn’t very easy. The FDA requires that you do it, but there are no specific instructions on how to do so other than following their long and complex list of guidelines. Some guidelines might not even apply in the case of your product. To help you out, we have written this blog which will help you with the SaMD (software as a medical device) validation process. Hence you need the help of a company like efour that is an expert in the field of medical software validation. We have a lot of experience in this topic here at efour, so please get in touch with us about your needs. Feel free to ask us anything you want. Let’s dig deep into the topic of validating medical device software.
What is Medical Software Validation?
First, we will have a look at what software validation means.
Software validation is a way to document, confirm and review whether the computer software you are using is
Medical device software validation is mandatory for all FDA-regulated companies. It helps you prove that the software can produce accurate and consistent results. The work maintains the level of compliance set by regulators. This helps them ensure quality control.
The FDA offers suggestions, but they don’t dictate what is considered “validation” for software. They also don’t say what the agreed-upon results should be like. Instead, it’s up to each company to show how they validate their software and provide evidence for this. Most companies typically buy medical software from a third party. But the company needs to handle medical software validation themselves. To handle the SaMD process, you should first ascertain whether you need to undergo the process. Let’s find out
Who Needs to Validate Medical Device Software?
You need to validate your software if your company falls under FDA regulations for medical devices.
This includes companies like
Adding more to this, understand that this is a non-comprehensive list. Please consult an expert to know whether you need to go for medical device software validation. But, validation is essential for any company that wants to improve quality, even if they’re not in a regulated industry. The FDA has a range of software validation tools that help companies with GxP and GMP procedures. With these, you can cut the risk and establish best practices for producing products.
The US FDA Software Validation Requirements

There are a few FDA requirements for medical device software validation. They are:
Companies need to provide validation plans to the FDA. These plans detail how the software will be used and provide a roadmap of what organizations need to do to approve its use. It also includes determining the specifications and quality standards.
The FDA has created general guidelines for validation projects, so they are not applicable to every company. As your product’s risk increases, the validation process will get more complicated.
For example, a pharmaceutical company that produces life-saving drugs is taking more risks than a manufacturer of products like vitamin C or sugar. Hence the validation approach for the medical device software of such a company will be more complex and stringent. Another case would be the use of IoT in healthcare. As the IoT infrastructure is more complex, it can attract stringent FDA validation norms.
A Standard Software Validation Method
The FDA and its guidance can seem tough to follow, but by taking the help of experts like efour, you can simplify this task.
Most validation projects follow the “4Q Lifecycle Model”. This model involves conducting tests and documenting the results. The 4Q model follows all the general principles of software validation. There are four stages in the 4Q model:
Design Qualification (DQ)
The software vendor can provide a script or instruction manual to document how to set up and use the product correctly. It might contain
Installation Qualification (IQ)
It’s important to assess if the software was correctly installed, and you can do that with tests and documentation. Understand that the software must meet your company’s specifications. It should also meet the user requirements along with the FDA’s guidance.
Operational Qualification (OQ)
We conduct medical device software testing at this stage. These tests help ensure that the software will consistently work as it’s meant to when operated in a range of standard use cases. These pre-launch tests will be provided by the vendors like efour, who are experts and know how their products should function. The OQ is an important part of the medical device software development process.
Performance Qualification (PQ)
This stage confirms that the software, as it was installed and configured, will perform the way your company needs it to. Your tests and documentation should show that the medical device verification and validation are complete. This shows that the software will properly be able to meet FDA standards of functionality and safety.
5 Steps to Software Validation
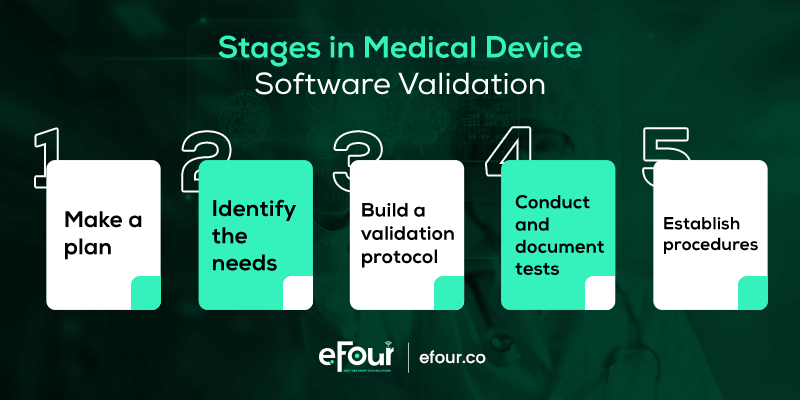
So how do you put this method into practice? Here is a step-by-step process to do so.
Step 1: Make a plan
Your validation plan should document and describe the following components.
Step 2: Determine the requirements of your system
For the medical device software to perform according to your expectations, your system must fulfill certain requirements. These include requirements such as
The SRS document helps in identifying any gaps in your functional requirements and inherent risks in your project. The SRS document also supports a risk analysis to identify unseen issues. What could go wrong? How can you moderate these inconveniences? Who is responsible for mitigating these risks? For instance, if a medical device malfunctions, that would be considered one category of risk. The severity of the occurrence and duration generally determine what steps, if any, should be taken to mitigate it.
Step 3: Build a validation protocol
Now you need to outline what the software does and what is the test method for validation of the medical device. A test plan documents why, what, and how it will be tested. This enables you to review the strengths and weaknesses of the testing plan. Your test cases should recreate common scenarios that prove your software can produce the proper outputs. They are designed to unearth as many errors as possible and prove whether key features are working properly.
Step 4: Conduct and document tests
Based on the test plan and carefully selected test cases, you will carry out the tests and document the results.
Step 5: Establish procedures
The last step is giving a final validation report, during which you detail how well the software performs. The test plan should also include info on support, training, security, backing up data and restoring it as and when necessary.
Software Validation Best Practices
What process you should follow will depend on what type of business you have, but there are best practices that can apply to most organizations. These include:
Amalgamate change management with software validation
Ensure that software validation automatically triggers every time a change happens. For example, when a system is installed, upgraded or updated. This helps you stay compliant and maintain standards like GxP or GMP. It also helps make sure that any change will not affect the safety of your product.
Test only those features that you will actually use
The validation process is a complex process. Hence you should save yourself time and effort by only testing the functionality that you will need as part of your production, inventory, or quality control system. When you’re done testing, make sure you disable any unused features, so users won’t come across them and waste time and effort.
Validate only the output of the software
You need to think about how your company plans to use the features, not the tools themselves. And you want to test whether your software can produce the desired outcomes as well as fit within FDA requirements. You are not just looking to make sure a certain feature works.
Rely on documentation provided by the vendors
One way to make your life easier is to use the validation documentation that most major software makers provide. These resources often offer a roadmap through the validation process and supply standard information. Software vendors can validate the way a product functions and promises to work for customers — in other words, the OQ stage. They can’t claim to know what the customer plans on doing with it later because that depends largely on policies and procedures (PQ).
FDA Software Validation Checklist
Through years of experience in providing medical software validation services to our clients, we at efour have prepared a medical device validation process. This is a template for validating medical device software. This template helps us to deliver effective results every single time.
Here is a sample medical software validation template that we follow.
Conclusion
Medical device software verification and validation is a tedious process. efour has highly qualified software engineers with years of experience. They’re trained in a variety of disciplines such as coding, testing, planning, verification, and validation of medical device software.
We ensure that your medical software conforms to all the medical device software standards. Our team includes expert engineers who have worked in the design and development of highly sophisticated medical devices at industry-leading companies. As a result, they have direct expertise in the validation of software for medical devices.
The development team of efour experts is well-equipped to handle any complex software validation activities for medical device makers. We can even handle IoT software development and validation. Contact us and leave your FDA software validation tensions on our strong shoulders.